Bi-Mask
Anatomically shaped anaesthesia face mask
Reusable
Soft and comfortable material
Latex free
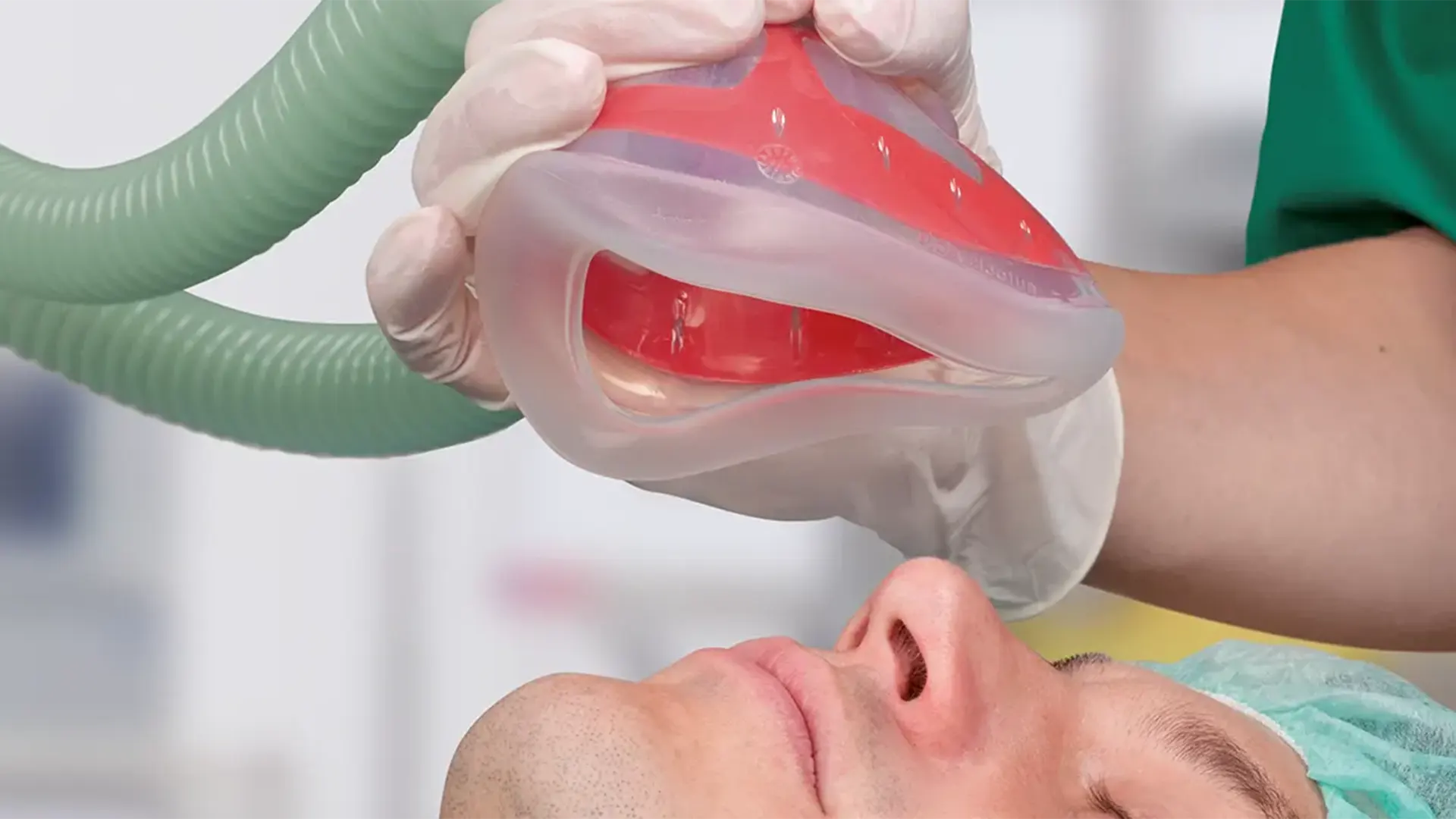
The Bi-Mask is a face mask made of silicone with integrated plastic shell. This supports a comfortable grip, as the mask is malleable and yet provides stability without collapse. Due to the anatomical shape, the Bi-Mask provides an airtight seal with minimum pressure and the ergonomical design allows an optimised grip via C-grip. The Bi-Mask is available in 5 sizes and processable up to 100 times (autoclavable at 134°C).
——Features

Mask body
Allows visibility for patient observation.
Internal plastic shell
- Provides stability without collapse
- Colour coding facilitates size selection
- Supports a comfortable grip due to its malleability
Soft silicone rim
Seals to a variety of face shapes.

22 mm I.D. connection
For resuscitator bag or breathing system.

UDI-Code and serial number
Product traceability and tracking of product usage.

Hook Ring
Allows fixation of the mask in combination with a fixation tape.

Sizes
The Bi-Mask is available in 5 sizes.
——Additional Information
——Related Topics & Products
Face Masks
WEB001_PID-01-05-03_AB_EN
Contact
VBM Medizintechnik GmbH
Einsteinstrasse 1
72172 Sulz a.N.
Germany
Tel.: +49 7454 9596-0
Fax: +49 7454 9596-33
e-mail: info@vbm-medical.de
www.vbm-medical.de
Directions</p
Technical Service
Medical Device Reporting
Cookie Settings
Change privacy settings
Privacy settings history
Revoke consents
Product Range
Cuff Pressure Gauges
Laryngeal Tube LTS-D
Cricothyrotomy
Resuscitators
Face Masks
Intubation Aids
Manual Suction Pump
Simulators
Pelvic Sling
Tube Fixations
Respiration Tubings
Catheter Mount Tubings
Connectors
Respiration Accessories
Pressure Infusion
Support Arms
Tube Connectors
Smooth Silicone Tubings
Electric Tourniquets
Compressed Air Tourniquets
Manual Tourniquets
Tourniquet Cuffs
Tourniquet Consumables
Company
The medical devices on this website are manufactured without the use of natural rubber latex, unless otherwise specified.
The medical devices on this website do not contain phthalates which require labelling according to CLP Regulation (EC) 1272/2008.
The VBM website includes information of the complete VBM medical device portfolio. Whether a medical device is available/approved in your region must be inquired. Please contact your VBM customer service.